Occuring:
This pathway is not found in animals; therefore, phenylalanine and tryptophan represent essential amino acids that must be obtained from the animals diet.
Different Steps involved Shikimic acid Pathway
Step 1: Phosphoenol pyruvate and erythrose-4-phosphate react to form 2-keto 3-deoxy 7-phospho glucoheptonic acid (DAHP), in a reaction catalyzed by the enzyme DAHP synthase.
Step 2: 2-keto3-deoxy 7-phosphogluco-heptonic acid (DAHP) is then transformed to 3-dehydroquinate (DHQ) or 3-Dehydroquinic acid, in a reaction catalyzed by DHQ synthase.
Although this reaction requires Nicotinamide Adenine Dinucleotide (NAD) as a cofactor, the enzymic mechanism regenerates it, resulting in the net use of no NAD.
Step 3: DHQ is dehydrated to 3-Dehydroshikimic acid by the enzyme 3-dehydroquinate dehydratase.
Step 4: which is reduced to shikimic acid by the enzyme shikimate dehydrogenase, which uses Nicotinamide Adenine Dinucleotide Phosphate (NADPH) as a cofactor.
Step 5: The next enzyme involved is shikimate kinase, an enzyme that catalyzes the ATP dependent phosphorylation of shikimate to form Shikimic acid 3-phosphate.
Step 6: Shikimic acid 3-phosphate is then coupled with phosphoenol pyruvate to give 5-enol pyruvyl shikimate -3- phosphate via the enzyme 5-enol pyruvyl shikimate-3-phosphate (EPSP) synthase.
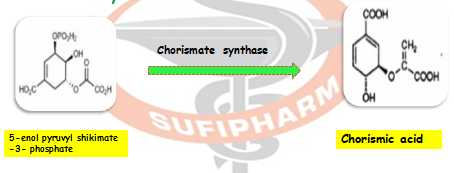
Step 7: Then 5-enolpyruvyl shikimate-3-phosphate is transformed into chorismic acid via the enzyme chorismate synthase.
Step 8, 9, 10: Prephenic acid is then synthesized by a Claisen rearrangement of chorismate by Chorismate Mutase.
Step 11, 12,13,14:
Prephenate is oxidatively decarboxylated with retention of the hydroxyl group by Prephenate dehydrogenase to give (para) p-hydroxyphenyl pyruvate, which is transaminated using glutamate as the nitrogen source to give tyrosine and a-ketoglutarate.
Pathway:
Starting Point in The Biosynthesis of Some Phenolic Phenyl alanine and tyrosine are the precursors used in the biosynthesis of phenyl propanoids.
The phenyl propanoids are then used to produce the flavonoids, coumarins, tannins and lignin.
Gallic acid biosynthesis Gallic acid is formed from 3-dehydroshikimate by the action of the enzyme shikimate dehydrogenase to produce 3,5-didehydroshikimate.
The latter compound spontaneously rearranges to gallic acid.
Other compounds
Shikimic acid is a precursor for:
Indole, indole derivatives and aromatic amino acid tryptophan and tryptophan derivatives such as the psychedelic compound dimethyl tryptamine. & many alkaloids and other aromatic metabolites.
Uses:
In the pharmaceutical industry, shikimic acid from the Chinese star anise (Illicium verum) is used as a base material for production of oseltamivir (Tamiflu).
Target for drugs:
Shikimate can be used to synthesize (6S)-6-Fluoro shikimic acid, an antibiotic which inhibits the aromatic biosynthetic pathway.
Glyphosphate, the active ingredient in the herbicide Roundup, kills plants by interfering with the shikimate pathway in plants. More specifically, glyphosate inhibits the enzyme 5-enolpyruvyl shikimate-3-phosphate synthase (EPSPS).
Roundup Ready genetically modified crops overcome that inhibit
Just click for download the lecture










0 Comments